Background and Objectives: Oral human papillomavirus (HPV) infection is the main cause of oropharyngeal cancer. However, there is no assessment tool for early detection and prevention of oropharyngeal cancer in practice. The purpose of the study was to develop and validate a risk assessment tool to predict the presence of HPV associated with oropharyngeal cancer.
Methods: Using data from the National Health and Nutrition Examination Survey 2011-2014, 6,978 US adults aged 18 to 59 years who were tested for oral HPV infection were included for this study. We carried out an analysis to test and validate risk predictive models for oral HPV infection. Presence of one of the 20 HPV subtypes associated with oropharyngeal cancer was used for the outcome.
Results: Of 6,978 participants aged 18-59, 303 (4.3%; 6.6 million) were found to have oncogenic HPV subtypes. Our final model included sex, income-to-poverty ratio, current smoking, and the lifetime number of oral sex partners. The discriminatory power of the oral HPV risk score to predict the presence of oncogenic HPV was good (C-statistic=0.73). The risk score performed comparably in the validation population (C-statistic=0.72). The comparison between observed and estimated proportions of population with oncogenic oral HPV demonstrated excellent calibration.
Conclusions: We developed and validated the oral HPV risk score that predicts the risk of oral HPV requiring only self-reported data and no laboratory testing. The Oral HPV risk score has the potential to provide clinicians with a no-cost, easy way to screen for patients at greater risk for oncogenic HPV infection.
Human papillomavirus (HPV) has been identified as a key cause in the development of oropharyngeal cancer.1 In fact, HPV is suggested to be the underlying cause of 70% of oropharyngeal cancers in the United States.2 Oral HPV is not uncommon.3 However, not all subtypes of HPV are linked to oropharyngeal cancer. One of the most common oncogenic oral HPV (subtype 16) is present in 3.5% of US adults.4 Moreover, although many of those infections clear, a substantial number of individuals have persistent infections.5
Many individuals do not have optimal access to dental services,6 the National Center for Health Statistics indicated that 35.6% of US adults aged 18 to 64 years did not see a dentist in 2016.7 Consequently, because of the relationship between oral health and systemic disease,8-10 primary care providers have become more aware of oral health screening and examinations. The Smiles for Life program is an example of a very successful oral health curriculum for primary care clinicians (https://smilesforlifeoralhealth.org). It includes oral health risk assessment tools. However, it does not include strategies to identify patients at high risk for oral HPV.
Currently, screening for oncogenic oral HPV would be challenging for several reasons. First, population-wide screening of all asymptomatic adults would be very inefficient, realizing that only a small proportion of the adult population is infected. Second, the cost of the test is not inconsequential at approximately $90 (https://www.lifelabs.com/how-to-order-hpv/), suggesting that targeted strategies for detection or increased surveillance for oropharyngeal cancer would be preferred. Further, the US Preventive Services Task Force recommendation on screening for oral cancer states that there is insufficient evidence to recommend screening in asymptomatic adults.11 With no specific guidance for screening for early detection and prevention of oropharyngeal cancer, it would be useful to identify the population most at risk for carriage of oncogenic HPV because of its outsized role in the development of oropharyngeal cancers. Thus, the purpose of this study was to develop a risk score for oncogenic oral HPV based on patient self-reported characteristics. We will develop and validate the risk score using nationally representative data for the United States. This goal is to create a practical and easily-implemented risk stratification tool that could identify individuals who may benefit from more frequent oral exams for prevention of oropharyngeal cancer.
Data and Sample
The development and validation of the oral HPV risk score was undertaken using data from adults aged 18-59 years, from the population-based National Health and Nutrition Examination Survey (NHANES), 2011-2014. The NHANES is a multistage probability sample of the civilian, noninstitutionalized US population. The NHANES includes physical exams, laboratory tests, and interviews with participating individuals. The Institutional Review Board of the University of Florida approved this study; the study was conducted in 2018.
Our initial unweighted sample included 9,853 participants in the NHANES mobile examination center (MEC). Participants more than 59 years old (n=1,786) were excluded from our risk score development because some questions about sexual behavior were not asked of these persons. Individuals who indicated that they had received the HPV vaccine were not included in the risk score development analysis (n=1,089). Although the current HPV vaccines do not cover all of the carcinogenic HPV subtypes, we felt that individuals who had received the HPV vaccine would be at very low risk, and so a risk score would not be useful for them. Based on these exclusion criteria, our final analytic sample included 6,978 US adults aged 18-59 years, representing population estimates for 153,886,760 Americans.
Measures
To be adopted into clinical practice, in addition to being valid, a prediction tool must be intuitive and conceptually simple.12 The conceptual basis for the oral HPV risk score is to build on information that is readily obtainable from the patient by self-report. We only examined predictors that could be self-reported or measured without obtaining a laboratory sample, because if a laboratory sample was required for computation, it would diminish the ease and value of the risk score in a clinical context.
Candidate Variables
We selected the candidate variables from risk factors for oral HPV identified from the literature.3,13 We identified the variables from the literature that met our criteria of being able to be assessed via patient self-report; we recoded the variables to simplify and result in meaningful categories prior to analysis. Sociodemographic characteristics included age (18-39 years and 40-59 years); sex (female and male); race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other); education (high school diploma or less and at least some college); marital status (married and not married), income-to-poverty ratio (IPR; <1.0 [in poverty] and ≥ 1.0 [not in poverty]). Concerning substance use, we included and used dichotomized measures of current smoking, marijuana use, and binge drinking. Current marijuana use was defined if a participant reported using marijuana or hashish during the past 30 days. Binge drinking was defined as consumption of at least five five drinks for male or at least four for female in 2 hours at least one occasion during the past 30 days.14 Sexual behavior information included lifetime number of oral sex partner regardless of gender orientation and history of any sexually transmitted infection (eg, genital warts, herpes, gonorrhea, and chlamydia). Oral sex partners was classified into two groups (0-7 partners and at least 8 partners) with the high group corresponding to the 75th percentile among the sample of adults.
Outcome Variable
The outcome variable is the presence of one of the 20 HPV subtypes associated with oropharyngeal cancer. We added eight more subtypes suggested by literature in addition to the group 1 carcinogens, resulting in the inclusion of HPV subtypes 6, 11, 16, 18, 31, 32, 33, 35, 39, 45, 51, 52, 53, 56, 57, 58, 59, 73, 81, and 82.15-17 Oral rinse specimens were processed, stored, and shipped to a central laboratory. Detailed specimen collection and processing instructions are discussed in the NHANES Laboratory/Medical Technologists Procedures Manual.18 Purified DNA was analyzed for 37 types of HPV by means of a multiplex polymerase-chain-reaction (PCR) assay. For the oral HPV risk score we classed as our outcome variable presence of one or more of the 20 HPV subtypes.
Data Analyses
Model and Risk Score Development. All analyses were conducted using SAS 9.4 (SAS Institute) so that we could make population estimates for the US noninstitutionalized adult population. We took the dataset based on the 2011-2014 NHANES and randomly split it into two equal databases. One database was used for model and score development to determine effectiveness of the new risk score classification in identifying individuals with carcinogenic oral HPV. The holdout sample was used for additional analysis to determine and validate whether, in a new sample, subjects can be correctly classified with respect to oral HPV.
We used multivariable logistic regression. Beginning with the full unrestricted model including all candidate variables, we used manual backwards elimination to determine the optimal set of candidate variables, which would best predict the presence of carcinogenic oral HPV. We treated missing values for the candidate variables (ranging from 2.9% for smoking to 13.8% for the lifetime number of oral sex partners) by listwise deletion in the models. We compared each step of variable exclusions (nested models) with the full model and preceding model using the negative of twice the log likelihood (-2 LL) and Aikaike Information Criteria (AIC). We reported odds ratios (ORs) and 95% confidence intervals (CIs) for the final multivariable model.
Converting the Model Results to Integer Risk Points. The measure is designed to be conceptually easy to grasp by clinicians and used in clinical settings as well as by patients on their own. For this reason, we rounded the resulting statistically significant odds ratios to whole number risk points, using the method of Charlson in developing the Charlson Comorbidity Index.19 Using this strategy, significant odds ratios between 1 and 1.19 scored 0 points; ratios from 1.2 to 1.49 were rounded to 1 point; ratios from 1.5 to 2.49 were rounded to 2 points, and so forth. The regressions were computed with the risk scores based on integer risk points. We computed C-statistics and evaluated to assess the discrimination and classification utility of the model.
Risk Score Validation. We computed the risk scores in the holdout sample used for validation, and we compared the resulting C statistics between the development sample and the validation sample. Discrimination and calibration in the validation sample was then tested by computing the C‐statistic and calibration slope and intercept (ie, a slope of 1 and an intercept of 0 indicates perfect calibration). The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive likelihood ratio (+LR), and negative likelihood ratio (-LR) were reported for various cutoff points of the final risk score.
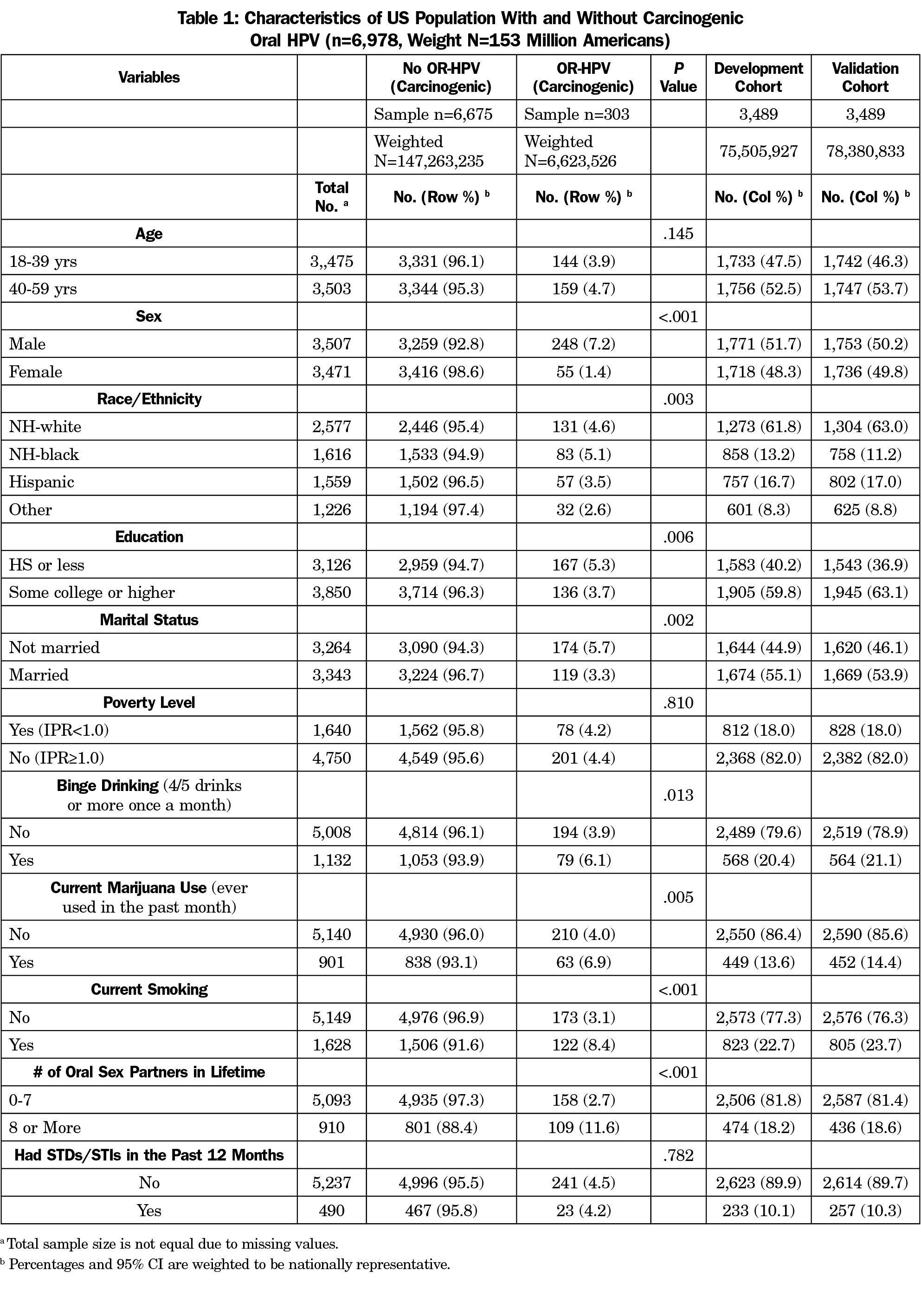
Among 6,978 participants aged 18-59 who were tested for oral HPV infection, 303 (4.3%; 6.6 million) were with the presence of HPV subtypes associated with oropharyngeal cancer. Table 1 shows the baseline characteristics of the participants with and without HPV subtypes associated with oropharyngeal cancer. Participants with HPV infection associated with oropharyngeal cancer were more likely to be male, non-Hispanic black, and had a poor health-related behavior profile (eg, current smoker, marijuana use, binge drinking, and greater number of oral sex partners). The oral HPV risk score was derived from the first 3,489 participants and validated from the second half. No significant differences across baselines characteristics were observed between the development and validation cohorts.
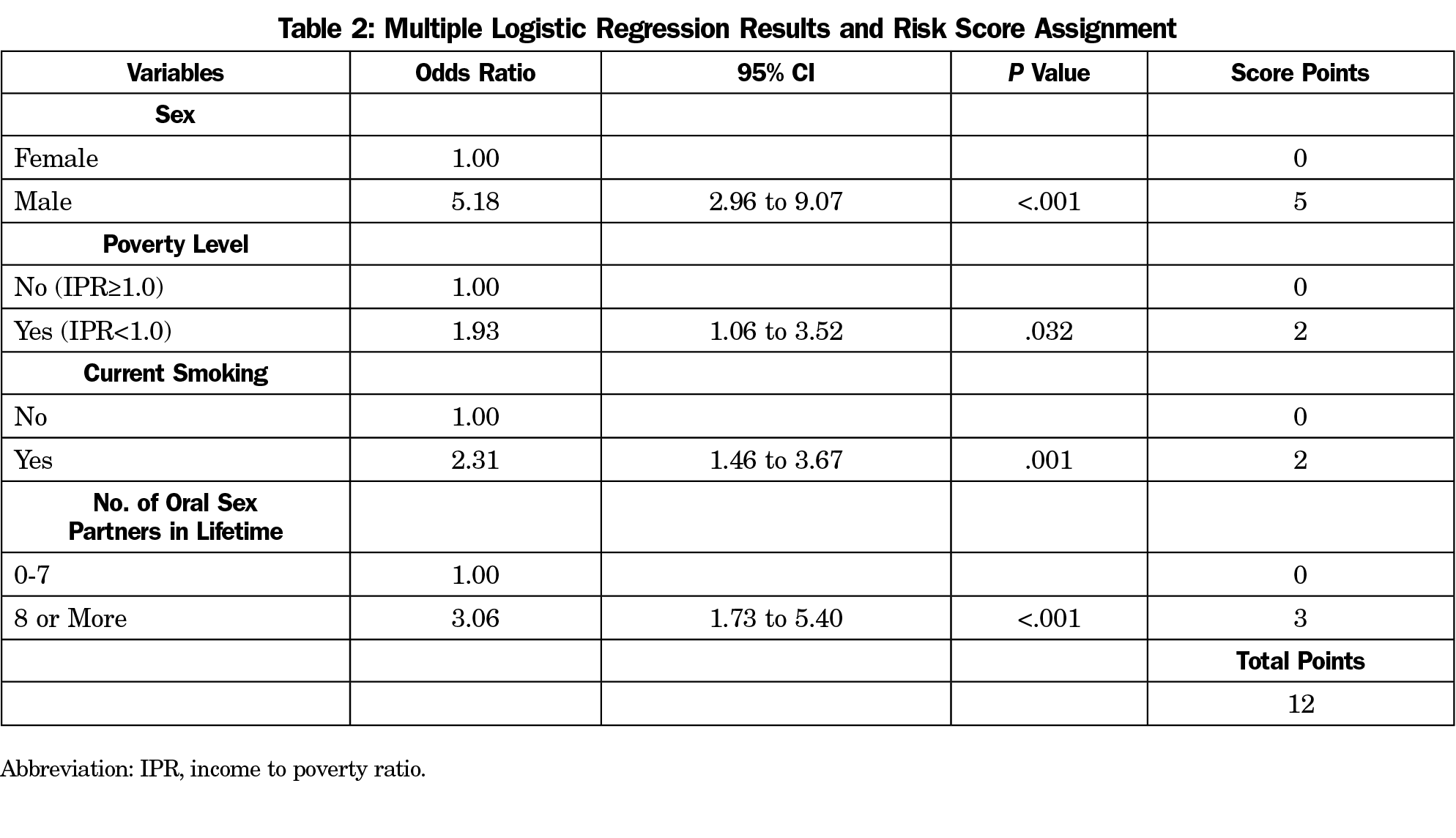
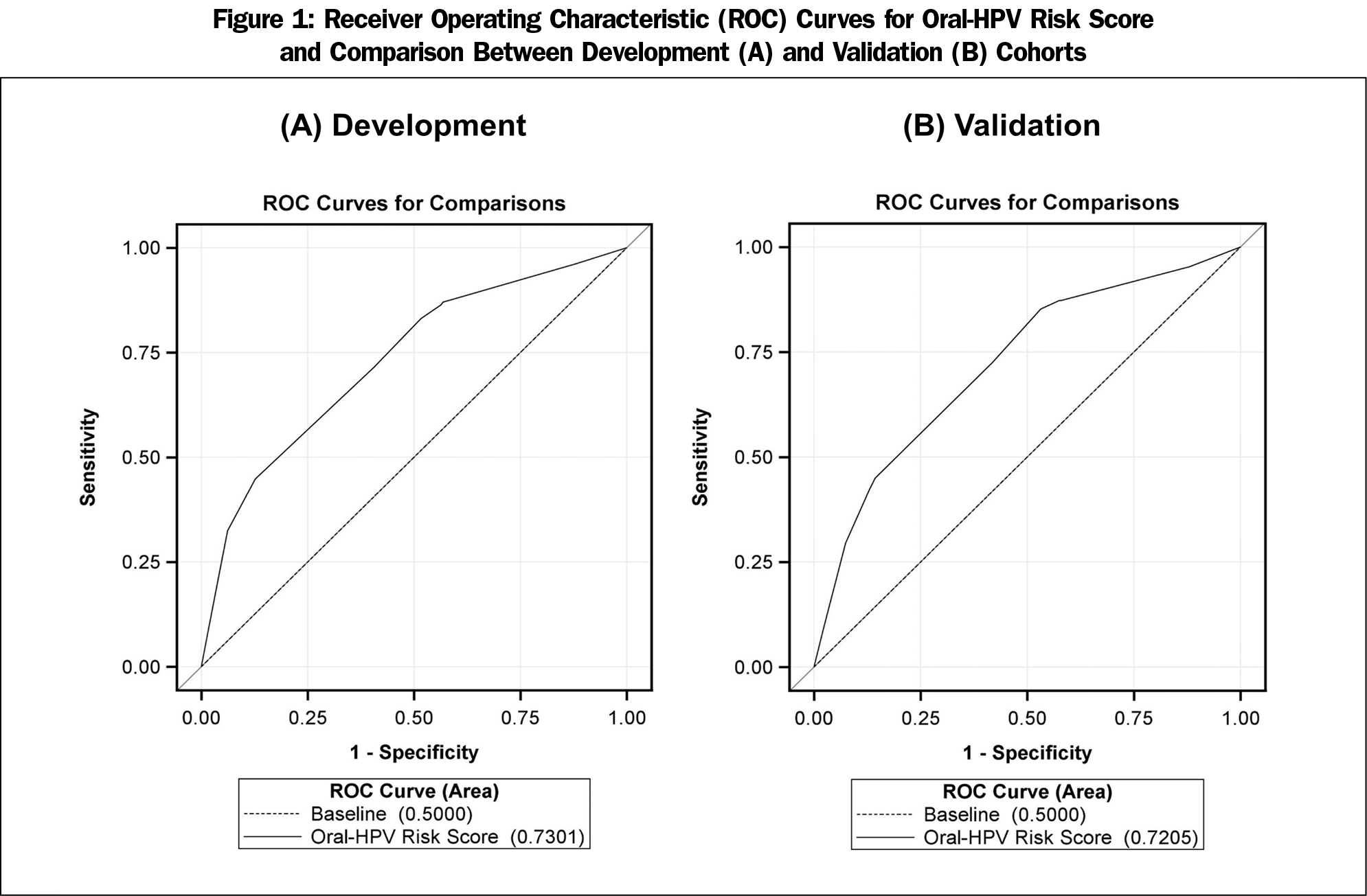
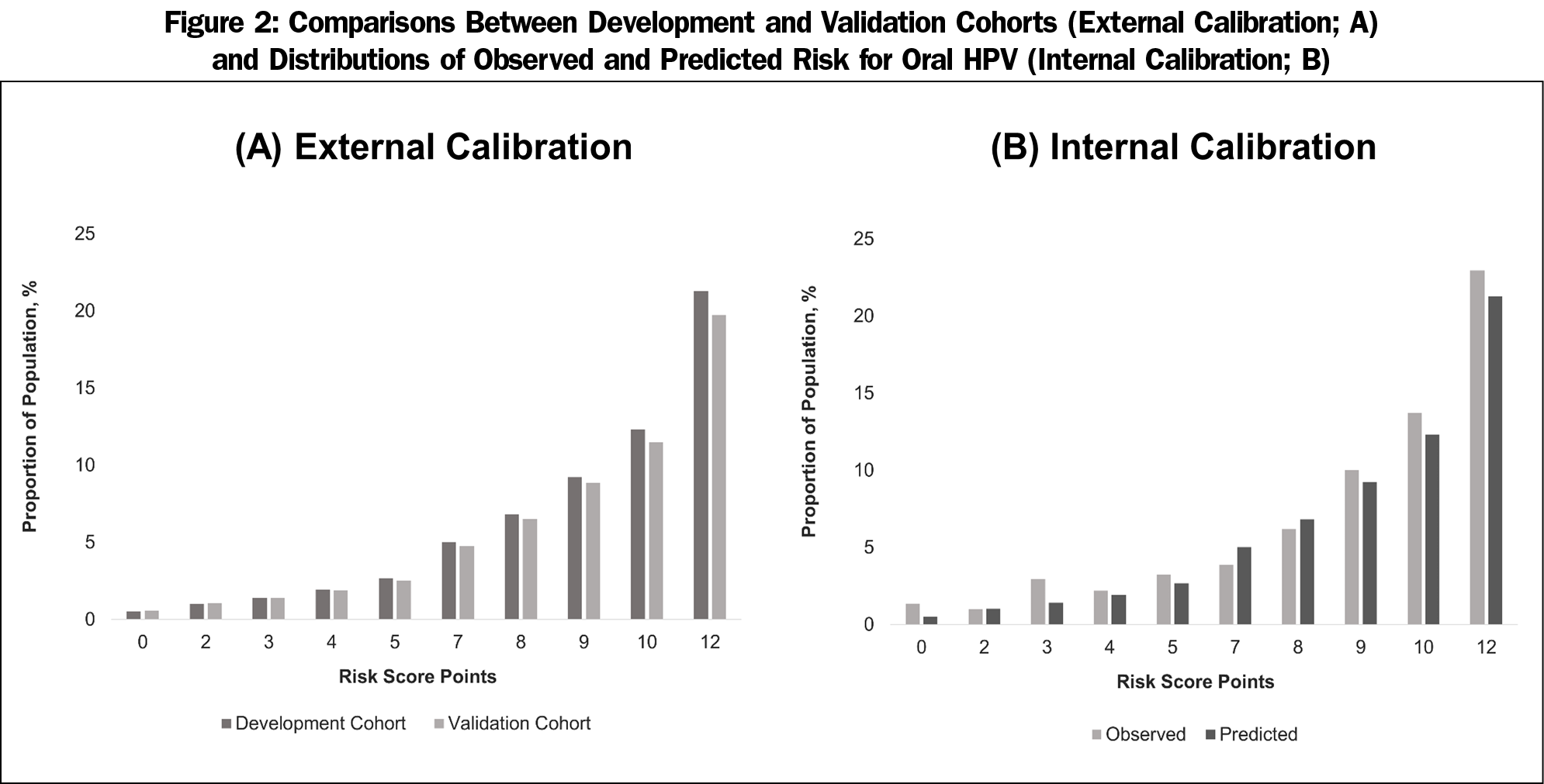
After the backward elimination of the nonsignificant candidate variables, the final best fit model included sex, IPR, current smoking, and the lifetime number of oral sex partners (Table 2). Being male scored 5 (OR, 5.18, 95% CI, 2.96-9.07), in poverty scored 2 (OR, 1.93, 95% CI, 1.06-3.52), current smoking scored 2 (OR, 2.31, 95% CI, 1.46-3.67), and having more than 7 lifetime oral sex partners scored 3 (OR, 3.06, 95% CI, 1.73-5.40). The area under the curve (AUC) for this model was 0.730 (C-statistic), indicating adequate discrimination (Figure 1-A). The performance of oral HPV risk score in the validation cohort was similar given C-statistic of 0.721. Figure 2 show the calibration plots for observed proportion of participants with oral HPV infection and predicted risks across the risk score points (Figure 2-A) and predicted risks between the development and validation cohorts (Figure 2-B). There was no evidence of risk overestimates with calibration slopes 1.06 and 1.03, respectively, suggesting that the developed score was well calibrated. The performance of the risk score at different cut-off points is present in Appendix Table 1 (https://journals.stfm.org/media/2805/mainous-appendixtable-fm52-1-2020.pdf). A score of at least 5 (85.9% vs 47.3%) or at least 7 (78.9% vs 56.8%) had a higher sensitivity, while a score of 8 (86.4% vs 57.0%) or greater cut-off points had a higher specificity than sensitivity. Cut-off points of at least 8 or 9 yielded comparable performance with 20% to 30% increased probabilities of oral HPV infection (ie, +LR of 2 increase probability 15%).20 For clinical use, different cutoff points may be chosen depending on screening purposes: high sensitivity for minimizing false negatives vs high specificity for minimizing false positives.
We developed and validated a simple integer-point risk score using a nationally representative cohort. This is the first study to create a risk score to identify those most at risk for oncogenic oral HPV, which would guide the screening for early detection and prevention of oropharyngeal cancer caused by HPV. The risk score predicts the risk of oral HPV requiring only self-reported data and no laboratory testing. Because of the use of only self-reported data and no required diagnostics, this risk score has potential utility in public health, health systems and self-evaluation settings with little to no financial cost and very little time required.
The value of this easily computed risk score is its widespread applicability that allows for risk stratification of individuals and appropriate surveillance for early detection of oropharyngeal cancer and targeted counseling efforts for behavioral modification of high-risk individuals. It can be particularly useful for guiding people who do not go to the dentist for routine oral examinations. The risk score can be administered in the primary care physician’s waiting room and other outpatient settings, or individuals can calculate their risk at home. After self-evaluation, if an individual finds himself in the high-risk category, he/she can be mindful of any mouth lesion that may appear and that takes longer to heal, and schedule an appointment with the dentist for a timely examination. Given the increasing emphasis on nondental health care providers in oral health care services,8-10,21 family physicians and primary care providers should be encouraged to play a role in oral cancer screening during their routine physical exams, including quick visual inspection (eg, oral cavity, tongue, and oropharynx for suspicious legions), oral health counseling, and further referrals based on the results of the screening. It is also critical for them to promote HPV vaccination uptake among age-eligible adolescents and young adults through patient education (including parents or guardians) to raise awareness of HPV-related health consequences.22
Considering the untreatable nature of HPV infection, unfavorable prognosis of oropharyngeal cancer, and the low cost and high potential benefits of screening for early detection and prevention, a score of 7 or higher (78.9% sensitivity and 56.8% specificity) may be chosen under the current context of the United States when there are no national guidelines for screening of oncogenic oral HPV. Nevertheless, when applied to a specific setting, it should be noted that the chosen threshold needs to be decided in consideration of other factors such as the prevalence of disease in the target population, the resources available, and the screening requirements.
Since the risk score was developed using a population-based nationally representative sample in the United States, its validity to be applicable nationwide increases. Also, we only used factors that were self-reported or measured without any laboratory test and converted each risk factor to simple integer scores, which makes its applicability simple, cost-effective, and easy in both clinical and self-evaluation settings. Additionally, this tool can also be administered to screen the population with lower utilization of dental care to improve referrals and routine follow-ups for surveillance.
Despite the many strengths of this study, some limitations need to be acknowledged. This risk score only examines the current risk and does not predict future risk since it has been developed from cross-sectional data. The C-statistics in both development and validation model is around 0.73, which is not the best; however, it is consistent with other similar cross-sectional risk scores developed to guide screening and surveillance.23-25 Although self-reported characteristics increase the applicability of this tool, self-report itself suffers from recall bias and social desirability, particularly around the sensitive topic of sexual behavior. Further, although the poverty-to-income ratio is a better indicator of economic status than household income because it includes both family size and the federal poverty threshold, it would be hard for patients to compute immediately. Providing some help in the clinic to the patient to tell them if they are above or below the poverty line may be necessary. The predictability of this risk score might be fluctuated among different oropharyngeal cancers due to the differential gradient of the strength of association between HPV and that specific cancer. Lastly, the current application of the tool is limited only to the population of the United States with ages between 18 and 59. For example, considering the median age of 63 for oropharyngeal cancer, further external validation to apply to those aged 60 or older is needed.
This is the first risk score that allows for risk stratification of adults for oncogenic oral HPV and can be applied in routine clinical practice for early detection of oropharyngeal cancer and targeted behavioral modification. Further research is warranted to investigate the timing and efficacy of screening in different settings that contribute to the validity of this risk score.
References
- Candotto V, Lauritano D, Nardone M, et al. HPV infection in the oral cavity: epidemiology, clinical manifestations and relationship with oral cancer. Oral Implantol (Rome). 2017;10(3):209-220. https://doi.org/10.11138/orl/2017.10.3.209
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294-4301. https://doi.org/10.1200/JCO.2011.36.4596
- Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009-2010. JAMA. 2012;307(7):693-703. https://doi.org/10.1001/jama.2012.101
- D’Souza G, McNeel TS, Fakhry C. Understanding personal risk of oropharyngeal cancer: risk-groups for oncogenic oral HPV infection and oropharyngeal cancer. Ann Oncol. 2017;28(12):3065-3069. https://doi.org/10.1093/annonc/mdx535
- Pierce Campbell CM, Kreimer AR, Lin HY, et al. Long-term persistence of oral human papillomavirus type 16: the HPV Infection in Men (HIM) study. Cancer Prev Res (Phila). 2015;8(3):190-196. https://doi.org/10.1158/1940-6207.CAPR-14-0296
- Sams LD, Rozier RG, Quinonez RB. Training Requirements and Curriculum Content for Primary Care Providers Delivering Preventive Oral Health Services to Children Enrolled in Medicaid. Fam Med. 2016;48(7):556-560.
- Center for Disease Control and Prevention. Oral and Dental Health. 2016. https://www.cdc.gov/nchs/fastats/dental.htm. Accessed October 25, 2019.
- Institute of Medicine and National Research Council. Improving Access to Oral Health Care for Vulnerable and Underserved Populations. Washington, DC: National Academies Press; 2011.
- Health Resources and Services Administration. Integration of Oral Health and Primary Care Practice. U.S. Department of Health and Human Services, Health Resources and Services Administration, February 2014. https://www.hrsa.gov/sites/default/files/hrsa/oralhealth/integrationoforalhealth.pdf. Accessed October 25, 2019.
- Association of American Medical Colleges. Report IX Contemporary Issues in Medicine: Oral Health Education for Medical and Dental Students. Washington DC: AAMC; June 2008.
- U.S. Preventive Services Task Force. Clinical Summary. Oral Cancer: Screening. https://www.uspreventiveservicestaskforce.org/Page/Document/ClinicalSummaryFinal/oral-cancer-screening1. Accessed December 14, 2018.
- Liao L, Mark DB. Clinical prediction models: are we building better mousetraps? J Am Coll Cardiol. 2003;42(5):851-853. https://doi.org/10.1016/S0735-1097(03)00836-2
- D’Souza G, Wentz A, Kluz N, et al. Sex Differences in Risk Factors and Natural History of Oral Human Papillomavirus Infection. J Infect Dis. 2016;213(12):1893-1896. https://doi.org/10.1093/infdis/jiw063
- National Institute on Alcohol Abuse and Alcoholism. Drinking Levels Defined: Binge Drinking. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking. Published 2018. Accessed January 1, 2019.
- Bouvard V, Baan R, Straif K, et al; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens—Part B: biological agents. Lancet Oncol. 2009;10(4):321-322. https://doi.org/10.1016/S1470-2045(09)70096-8
- Kim SM. Human papilloma virus in oral cancer. J Korean Assoc Oral Maxillofac Surg. 2016;42(6):327-336. https://doi.org/10.5125/jkaoms.2016.42.6.327
- Shigeishi H, Sugiyama M. Risk Factors for Oral Human Papillomavirus Infection in Healthy Individuals: A Systematic Review and Meta-Analysis. J Clin Med Res. 2016;8(10):721-729. https://doi.org/10.14740/jocmr2545w
- Centers for Disease Control and Prevention. NHANES Laboratory/Medical Technologists Procedures Manual. 2013. https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/manuals/2013_MEC_Laboratory_Procedures_Manual.pdf. Accessed January 7, 2018.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. https://doi.org/10.1016/0021-9681(87)90171-8
- McGee S. Simplifying likelihood ratios. J Gen Intern Med. 2002;17(8):646-649. https://doi.org/10.1046/j.1525-1497.2002.10750.x
- Cohen LA. Expanding the physician’s role in addressing the oral health of adults. Am J Public Health. 2013;103(3):408-412. https://doi.org/10.2105/AJPH.2012.300990
- American Academy of Pediatric Dentistry Policy on human papilloma virus vaccinations. Chicago: AAPD; 2017. http://www.aapd.org/media/Policies_Guidelines/P_HPV_Vaccinations.pdf. Accessed October 25, 2019.
- Barengo NC, Tamayo DC, Tono T, Tuomilehto J. A Colombian diabetes risk score for detecting undiagnosed diabetes and impaired glucose regulation. Prim Care Diabetes. 2017;11(1):86-93. https://doi.org/10.1016/j.pcd.2016.09.004
- Haroon S, Adab P, Riley RD, Fitzmaurice D, Jordan RE. Predicting risk of undiagnosed COPD: development and validation of the TargetCOPD score. Eur Respir J. 2017;49(6):1602191. https://doi.org/10.1183/13993003.02191-2016
- Carrillo-Larco RM, Miranda JJ, Gilman RH, et al; CRONICAS Cohort Study Group. Risk score for first-screening of prevalent undiagnosed chronic kidney disease in Peru: the CRONICAS-CKD risk score. BMC Nephrol. 2017;18(1):343. https://doi.org/10.1186/s12882-017-0758-4


There are no comments for this article.