Background and Objectives: Current strategies for obesity management in primary care leave many patients inadequately treated or unable to access treatment entirely. We aimed to evaluate a comprehensive, primary care clinic-based weight management program’s clinical effectiveness in a community practice setting.
Methods: This was an 18-month pre/postintervention study. We collected demographic and anthropometric data on patients enrolled in a primary care-based weight management program. The primary outcomes were percent weight loss postintervention and the proportion of patients who achieved a clinically significant total body weight loss (TBWL) of 5% or greater.
Results: Our program served 550 patients over 1,952 visits from March 2019 through October 2020. A total of 209 patients had adequate program exposure, defined as four or more completed visits. Among these, all received targeted lifestyle counseling and 78% received antiobesity medication. Patients who attended at least four visits had an average TBWL of 5.7% compared to an average gain of 1.5% total body weight for those with only one visit. Fifty-three percent of patients (n=111) achieved greater than 5% TBWL, and 20% (n=43) achieved greater than 10% TBWL.
Conclusion: We demonstrated that a community-based weight management program delivered by obesity medicine-trained primary care providers effectively produces clinically significant weight loss. Future work will include wider implementation of this model to increase patient access to evidence-based obesity treatments in their communities.
Obesity affects 42% of US adults, with persons of low socioeconomic status disproportionately being burdened.1, 2 Unfortunately, most treatment still occurs in centralized multidisciplinary centers or commercial clinics with high out-of-pocket costs. 3
As most health care interactions occur in primary care practices, providing weight management services in the same locations enhances patient access to obesity care and is expected to improve health outcomes.4, 5 However, the time constraints in primary care practices and lack of obesity-specific training are major barriers to obesity treatment in communities.6-10 Moreover, clinical guidelines underscore that intense behavioral therapy alone is insufficient to achieve sustained weight loss for most patients, necessitating the consideration of antiobesity medication (AOM) when bariatric surgery is declined. 11-15
To increase access, we piloted a weight management program (WMP) utilizing obesity medicine-trained primary care providers (PCPs) nested in primary care practices. We sought to evaluate the effectiveness of such a nested WMP for clinically meaningful weight loss in community-based practices as a scalable model for delivering comprehensive obesity care.
Setting
The WMP was piloted in three primary care clinics. Three PCPs certified in weight management by the American Board of Obesity Medicine were assigned visits dedicated to obesity care, charging primary care copays. Staffing included registered dieticians (RD) and licensed clinical social workers (LCSW) as part of a primary care medical home. All clinical staff were existing employees. We informed primary care physicians at the three pilot sites of our study. Physicians then identified patients and referred them to our weight management pilot program.
Intervention
The physician and RD evaluated all new patients. Evaluations included weight history, eating behaviors, physical activity, sleep, stress, physical exam, and metabolic data. Patients with uncontrolled mental health diagnoses were referred to LCSW. When AOMs were initiated, preference was given to FDA-approved agents. Generic medication combinations were prescribed to reduce cost when possible.
The program offered targeted lifestyle counseling (TLC; 6-8 sessions per year) plus AOMs. Physicians individualized interventions by applying the American Association of Clinical Endocrinology’s guidelines for obesity management.16 Providers offered bariatric surgery to patients meeting criteria.17 Patients received nutrition instructions emphasizing reducing ultraprocessed foods and following a Mediterranean-style diet rather than restricting calories due to extensive literature supporting the diet’s positive impact on cardiometabolic risk factors and overall health.18-20 This approach has been shown to reduce total caloric intake, reduced the complexity of dietary counseling, and reflected general patient and professional preference.18 Providers used motivational interviewing techniques to encourage patient-centered SMART goals for dietary choices, physical activity, stress management, and sleep improvement.21, 22
The obesity-certified PCPs were involved in every follow-up visit. Visits were 20 minutes in duration. The visits were primarily structured to address lifestyle changes. Goals were reviewed, positive behaviors were reinforced, and strategies were discussed to work on unmet goals. We also titrated antiobesity medications. Since weight loss has a profound impact on so many comorbidities, we regularly addressed significant conditions, such as hypertension, nonalcoholic fatty liver disease, obstructive sleep apnea, and type 2 diabetes, and titrated down or removed cardiometabolic medications. We offered in-person and virtual visits.
The program maintained financial stability for the clinics. All three clinicians were able to meet their goals for standard work relative value units (wRVU). The physicians used time-based, outpatient Current Procedural Terminology (CPT) codes for evaluation and management (E/M) services, the RDs used medical nutrition therapy codes, and the LCSW used behavioral health codes.
Evaluation Methods
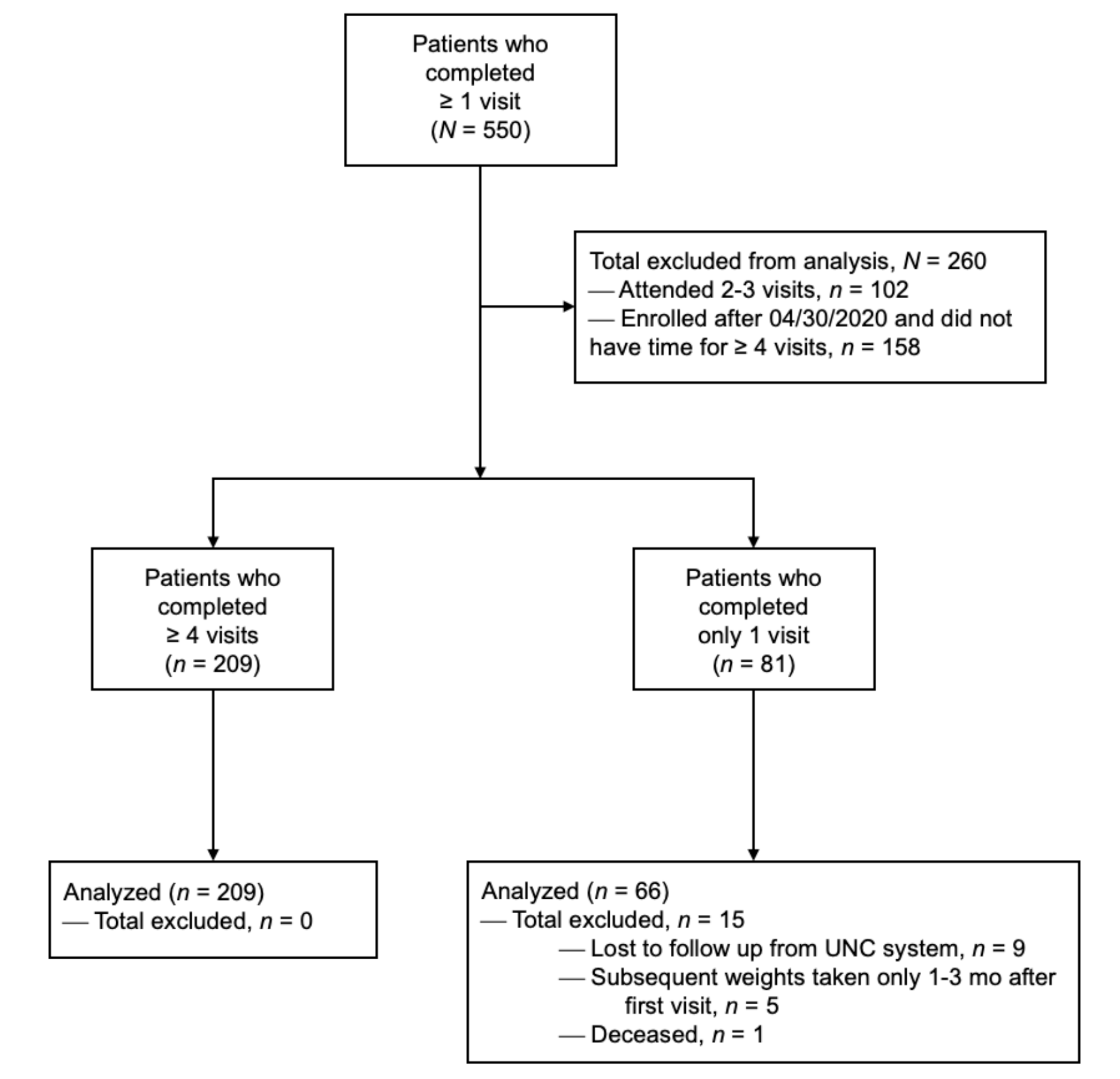
Patients enrolled in the WMP were tracked in an obesity registry. Health and demographic data were extracted and analyzed for patients 18 or older with body mass indexes (BMIs) above 27 kg/m2 and adequate program exposure, defined as at least four visits.23 BMI inclusion was based on current practice guidelines.16 The comparison group was patients with similar age and BMI criteria (Table 1) but only one assessment visit in the WMP and no follow-up visits or interventions. The study excluded patients who had had bariatric surgery within 6 months of the study period (Figure 1).
|
Characteristic
|
Total Patients, N=550 a
|
Four or More Visits, N=209
|
Only One Visit, N=66
|
|
Age, mean (SD), year
|
49.0 (13.3)
|
49.2 (12.8)
|
49.3 (13.0)
|
|
BMI, mean (SD), kg•m -2
|
40.0 (7.7)
|
40.1 (7.4)
|
39.1 (8.0)
|
|
Sex – no. of patients (%)
|
|
|
|
|
Female
|
464 (84.4%)
|
179 (85.7%)
|
57 (86.4%)
|
|
Race – no. of patients (%)
|
|
|
|
|
White/Caucasian
|
343 (62.4%)
|
132 (63.2%)
|
34 (51.5%)
|
|
Black/African American
|
164 (29.8%)
|
61 (29.2%)
|
26 (39.4%)
|
|
Asian
|
7 (1.3%)
|
4 (1.9%)
|
0 (0)
|
|
American Indian or Alaska Native
|
1 (0.2%)
|
0 (0)
|
0 (0)
|
|
Native Hawaiian Or Other Pacific Islander
|
1 (0.2%)
|
0 (0)
|
0 (0)
|
|
Other race
|
21 (3.8%)
|
9 (4.3%)
|
1 (1.5%)
|
|
Unknownb
|
13 (2.3%)
|
3 (1.4%)
|
5 (7.6%)
|
|
Ethnicity – no. of patients (%)
|
|
|
|
|
Non-Latino or Hispanic
|
504 (91.6%)
|
190 (91.0%)
|
60 (90.9%)
|
|
Latino or Hispanic
|
25 (4.6%)
|
12 (5.7%)
|
3 (4.5%)
|
|
Unknownb
|
21 (3.8%)
|
7 (3.4%)
|
3 (4.5%)
|
|
Comorbidities – no. of patients (%)
|
|
|
|
|
Hypertension
|
264 (48.0%)
|
99 (47.4%)
|
36 (54.5%)
|
|
Depression
|
232 (42.2%)
|
98 (46.9%)
|
21 (31.8%)
|
|
Obstructive sleep apnea
|
135 (24.5%)
|
40 (19.1%)
|
16 (24.2%)
|
|
Diabetes mellitus
|
96 (17.5%)
|
34 (16.3%)
|
16 (24.2%)
|
|
Polycystic ovarian syndrome
|
17 (3.1%)
|
10 (4.8%)
|
2 (3.0%)
|
|
Nonalcoholic fatty liver disease
|
35 (6.4%)
|
13 (6.2%)
|
6 (9.1%)
|
|
Nonalcoholic steatohepatitis
|
5 (0.9%)
|
2 (1.0%)
|
0 (0)
|
|
Cirrhosis of liver
|
2 (0.4%)
|
1 (0.5%)
|
1 (1.5%)
|
|
Payor Types – no. of patients (%)
|
|
|
|
|
Commercialc
|
373 (67.8%)
|
151 (72.2%)
|
43 (65.2%)
|
|
Medicare
|
88 (16.0%)
|
30 (14.4%)
|
15 (22.7%)
|
|
Medicaid
|
60 (10.9%)
|
14 (6.7%)
|
6 (9.1%)
|
|
Uninsuredd
|
29 (5.3%)
|
14 (6.7%)
|
2 (3.0%)
|
The primary outcomes were the percent weight loss postintervention and the proportion of patients who achieved a clinically significant TBWL of 5% or greater. We analyzed the data using Stata/SE software version 16.1.24 We performed paired t tests to assess differences in continuous variables. We established statistical significance at ɑ=0.05. We compared the latest weight and BMI to the baseline values at the program's start to determine changes in outcomes. The University of North Carolina at Chapel Hill’s Institutional Review Board approved the study.
From March 2019 through October 2020, the WMP’s three pilot clinics served 550 new patients over 1,925 visits. The mean patient age was 49.0 years (SD 13.3), with a starting weight and BMI of 110.2 kg (SD 23.5) and 40.0 kg/m2 (SD 7.7), respectively (Table 1).
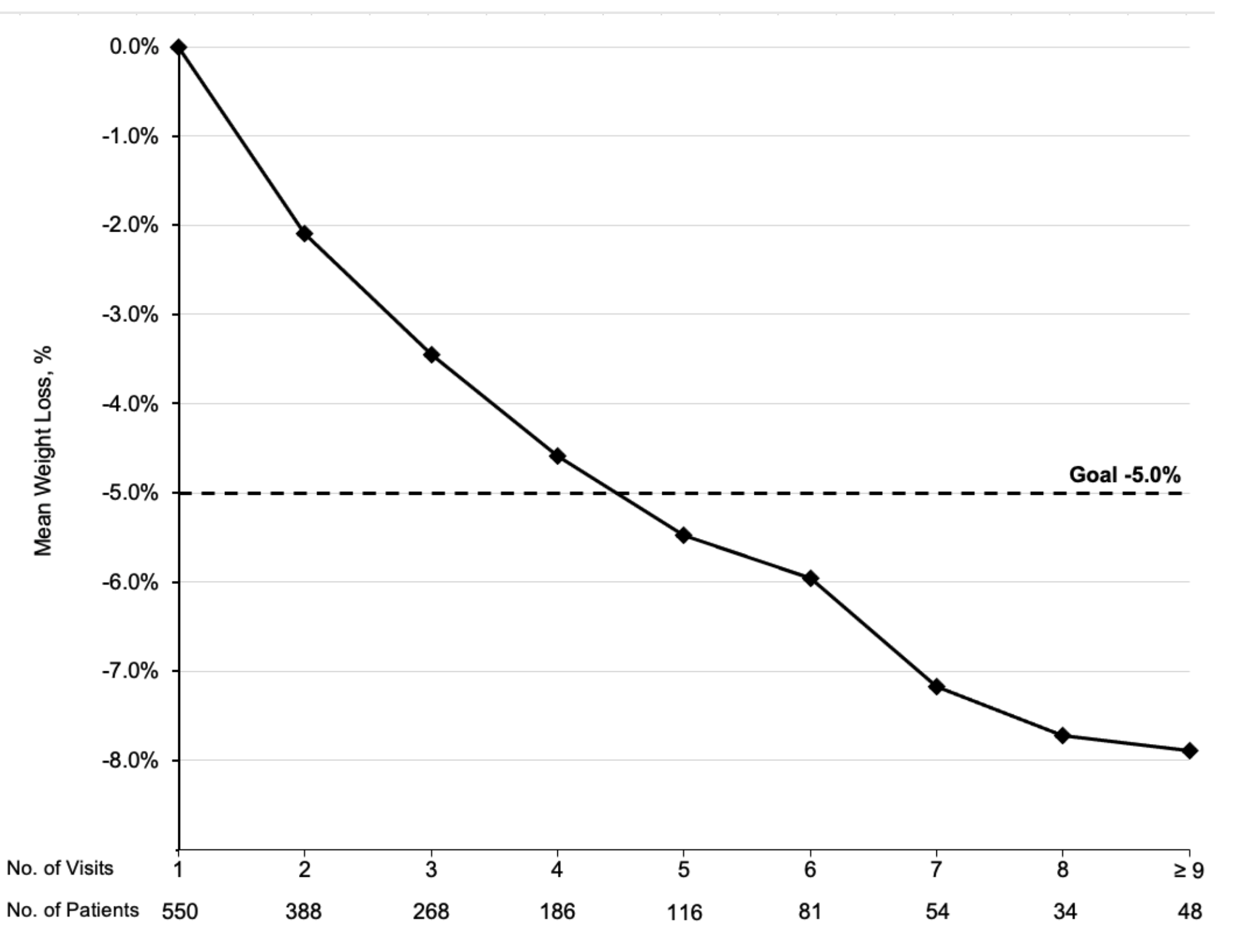
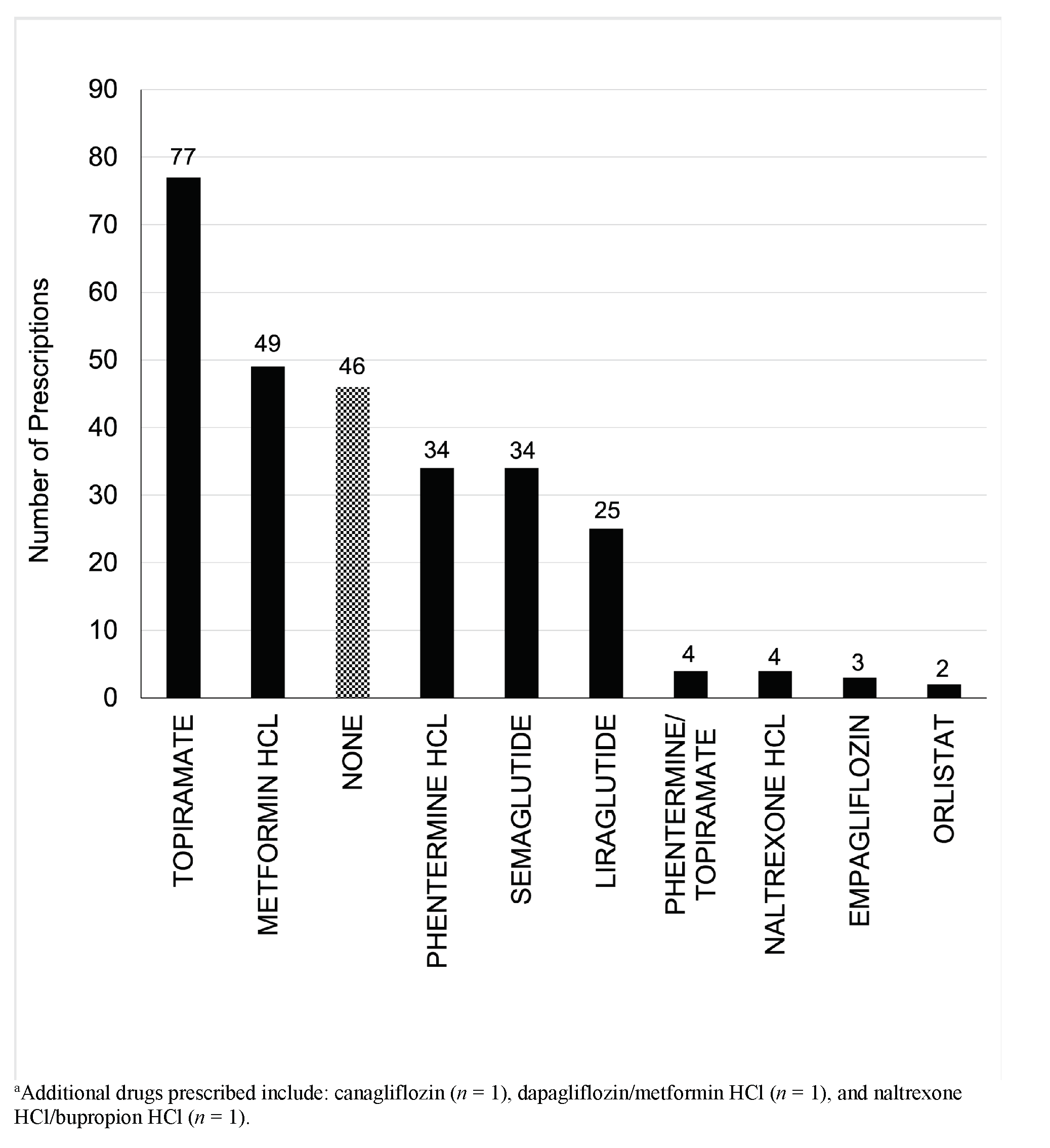
For inclusion in the analysis, 209 patients had adequate program exposure (≥4 visits) and 66 patients were in the comparison group (assessment without follow-up). The mean weight change was -5.7% (SD 5.8%), average attendance was 6.3 visits (SD 2.6), and mean follow-up was 241 days (SD 127). Fifty three percent (n=111) achieved at least 5% TBWL with 6.8 mean visits. Twenty percent of patients achieved 10% TBWL or more. The comparison group had a mean weight gain of 1.5% over an average of 407 days (SD 158; Table 2). The weight loss appeared to co-relate with the number of visits completed, with patients achieving TBWL of at least 5% between the fourth and fifth visit (Figure 2). AOM utilization is shown in Figure 3.
|
|
Adequate Exposure Group, N=209 Mean (SD or 95% CI)
|
Comparison Group, N=66 Mean (SD or 95% CI)
|
|
Pre BMI (kg•m-2)
|
40.1 (SD 7.4, range 28.0,75.6)
|
39.1 (SD 8.0, range 25.7,60.0)
|
|
Post BMI (kg•m-2)
|
37.8 (SD 7.3, range 23.3,72.6)
|
39.4 (SD 8.3, range 25.9,59.0)
|
|
Change in BMI
|
-2.3 (-2.6,-2.0; P <.001)
|
0.3 (-0.9,0.3; P =.33)
|
|
Preweight (kgs)
|
110.2 (SD 23.3, range 66.5,199.1)
|
107.4 (SD 26.7, range 60.3,194.3)
|
|
Postweight (kgs)
|
103.9 (SD 22.6, range 61.7,193.4)
|
109.2 (SD 27.0, range 59.6,199.1)
|
|
Change in weight
|
-6.3 (- 7.2,-5.5; P<.001)
|
1.8 (0.4,3.1; P<.01)
|
|
Percent change in weight
|
-5.7% (SD 5.8%, range -22.8%, 13.7%)
|
1.5% (SD 4.9%, range -7.9%, 14.7%)
|
|
≥5% weight loss
|
53.1% (n = 111)
|
7.6% (n = 5)
|
|
≥7% weight loss
|
38.8% (n = 81)
|
3.0% (n = 2)
|
|
≥10% weight loss
|
20.6% (n = 43)
|
0% (n = 0)
|
The purpose of our WMP was to explore the impact of obesity medicine-trained PCPs on durable weight loss in our communities. During the WMP pilot, our patients achieved a clinically meaningful weight loss of approximately 5% after attending at least four visits. Half of the patients (53%) achieved 5% weight loss compared to the marginal weight gain in individuals who only received the initial assessment. These results make our community-based WMP on par with programs in more controlled, resource-intense settings,25, 26 and did not require high-intensity counseling (>2 sessions/month) or referrals to specialty-based WMPs, which the current literature suggests are necessary for achieving such weight loss in primary care.6 We believe that our model’s leverage of obesity-trained PCPs in primary care practices can increase access to weight management services and meaningful weight loss among socioeconomically diverse patient populations.
There is growing evidence that moderate weight loss can significantly improve outcomes of many obesity-related comorbidities.27, 28 Accordingly, we believe that obesity treatment strategies can and should include community-based solutions to improve access to care and improve obesity-related outcomes. Increased access through primary care-based services has the potential to address specialty-based WMPs’ insufficient capacity within our health care system to address the rapidly increasing prevalence of obesity.
This study's strength is its achievement of significant weight loss in real-world community settings serving patients from diverse socioeconomic and underrepresented racial groups. Limitations of this study include its initiation as a quality improvement project with a small number of patients; the lack of a control group; its underrepresentation of males and Latino ethnicity; limited AOM insurance coverage; and the COVID-19 pandemic beginning midway through the study.
We do not think the weight management certification of the physicians is a critical component of the intervention. We have found that having easy access to obesity medicine-certified mentors is the key to providing effective, evidence-based obesity treatment in low-resource community settings. For example, at our institution, we used an information dissemination technique similar to the ECHO (Extension of Community Healthcare Outcomes) Model.29 We have monthly, virtual obesity medicine meetings with community clinicians discussing the latest obesity literature and difficult clinical cases. These meetings also build relationships such that PCPs can have access to experienced obesity clinicians. Also, our institution is sponsoring a local obesity conference for primary care professionals to teach effective weight management tools to be used by them in the community setting.
In conclusion, this approach represents a potential scalable model for expanding access to comprehensive, effective obesity treatment for the general population. The next steps include comparing community-based results to matched controls and to specialty-based obesity clinic outcomes at our institution.
Acknowledgments
We thank Darren DeWalt, MD, MPH; John Batsis, MD; and Young E. Whang, MD, PhD, for reviewing and editing the manuscript. We also want to thank Sachin Gupta, MD; Shawn Armstrong; Ellen Lawrence, RD; and Candice Hunt for help in implementing this program.
Financial Support
Program evaluation support was provided by a training grant from the UNC Institute for Healthcare Quality Improvement.
Presentations
This project was presented orally and as a poster at the Obesity Medicine Association’s Overcoming Obesity Fall 2020 Conference (Virtual) on October 7, 2020.
Conflict Disclosure
Dr Machineni reports personal fees and others from Novo Nordisk and personal fees from Rhythm Pharmaceuticals outside the submitted work. Dr Davis reports personal fees (speaking fees) from Novo Nordisk outside of the submitted work.
References
-
-
-
Krueger PM, Reither EN. Mind the gap: race/ethnic and socioeconomic disparities in obesity. Curr Diab Rep
. 2015;15(11):95. doi:10.1007/s11892-015-0666-6
-
Ashman JJ, Rui P, Okeyode T. Characteristics of office-based physician visits. NCHS Data Brief. 2016;(331):1-8.
-
Knowler WC, Barrett-Connor E, Fowler SE, et al; Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med
. 2002;346(6):393-403. doi:10.1056/NEJMoa012512
-
Tronieri JS, Wadden TA, Chao AM, Tsai AG. Primary care interventions for obesity: review of the evidence. Curr Obes Rep
. 2019;8(2):128-136. doi:10.1007/s13679-019-00341-5
-
Petrin C, Kahan S, Turner M, Gallagher C, Dietz WH. Current practices of obesity pharmacotherapy, bariatric surgery referral and coding for counselling by healthcare professionals.
Obes Sci Pract. 2016;2(3):266-271.
doi:10.1002/osp4.53
-
Petrin C, Kahan S, Turner M, Gallagher C, Dietz WH. Current attitudes and practices of obesity counselling by health care providers. Obes Res Clin Pract
. 2017;11(3):352-359. doi:10.1016/j.orcp.2016.08.005
-
Turner M, Jannah N, Kahan S, Gallagher C, Dietz W. Current knowledge of obesity treatment guidelines by health care professionals. Obes (Silver Spring).2018. 26:665–671 doi:10.1002/oby.22142
-
-
Hamman RF, Wing RR, Edelstein SL, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102-2107. doi:10.2337/dc06-0560
-
Wing RR, Hamman RF, Bray GA, et al; Diabetes Prevention Program Research Group. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obes Res
. 2004;12(9):1426-1434. doi:10.1038/oby.2004.179
-
Orchard TJ, Temprosa M, Barrett-Connor E, et al; Diabetes Prevention Program Outcomes Study Research Group. Long-term effects of the Diabetes Prevention Program interventions on cardiovascular risk factors: a report from the DPP Outcomes Study. Diabet Med
. 2013;30(1):46-55. doi:10.1111/j.1464-5491.2012.03750.x
-
Knowler WC, Fowler SE, Hamman RF, et al; Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet
. 2009;374(9702):1677-1686. doi:10.1016/S0140-6736(09)61457-4
-
Safavi R, Lih A, Kirkpatrick S, Haller S, Bailony MR. Impact of anti-obesity medication initiation and duration on weight loss in a comprehensive weight loss programme.
Obes Sci Pract. 2019;5(5):468-478.
doi:10.1002/osp4.361
-
Garvey WT, Mechanick JI, Brett EM, et al; Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract
. 2016;22(3)(suppl 3):1-203. doi:10.4158/EP161365.GL
-
Schauer PR, Bhatt DL, Kirwan JP, et al; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med
. 2017;376(7):641-651. doi:10.1056/NEJMoa1600869
-
Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake.
Cell Metab. 2019;30(1):67-77.e3.
doi:10.1016/j.cmet.2019.05.008
-
Williams LT, Barnes K, Ball L, Ross LJ, Sladdin I, Mitchell LJ. How effective are dietitians in weight management? a systematic review and meta-analysis of randomized controlled trials. Healthcare (Basel)
. 2019;7(1):20. doi:10.3390/healthcare7010020
-
Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr
. 2010;92(5):1189-1196. doi:10.3945/ajcn.2010.29673
-
Greenwald A. Current nutritional treatments of obesity. Adv Psychosom Med
. 2006;27:24-41. doi:10.1159/000090961
-
Whitehead L, Glass CC, Abel SL, Sharp K, Coppell KJ. Exploring the role of goal setting in weight loss for adults recently diagnosed with pre-diabetes. BMC Nurs
. 2020;19(1):67. doi:10.1186/s12912-020-00462-6
-
-
-
Pantalone KM, Smolarz BG, Ramasamy A, et al. Effectiveness of combining antiobesity medication with an employer-based weight management program for treatment of obesity: a randomized clinical trial. JAMA Netw Open. 2021;4(7):e2116595.
doi:10.1001/jamanetworkopen.2021.16595
-
Ikramuddin S, Korner J, Lee W-J, et al. Lifestyle intervention and medical management with vs without Roux-en-Y gastric bypass and control of hemoglobin A1c, LDL cholesterol, and systolic blood pressure at 5 years in the diabetes surgery study. JAMA
. 2018;319(3):266-278. doi:10.1001/jama.2017.20813
-
Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet
. 2018;391(10120):541-551. doi:10.1016/S0140-6736(17)33102-1
-
Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr
. 2012;95(2):297-308. doi:10.3945/ajcn.111.024927
-
Mendizabal M, Ridruejo E, Ceballos S, et al; Latin American Liver Research, Educational, Awareness Network (LALREAN). The ECHO model proved to be a useful tool to increase clinicians’ self-effectiveness for care of patients with Hepatitis C in Argentina. J Viral Hepat
. 2019;26(11):1284-1292. doi:10.1111/jvh.13172


There are no comments for this article.