Introduction: The incidence of melanoma is on the rise. In trained hands, dermoscopy aids in the differentiation of melanoma from benign skin growths, including melanocytic nevi. This study evaluated the impact of dermoscopy training for primary care practitioners (PCPs) on the number of nevi needed to biopsy (NNB) to detect a melanoma.
Methods: We conducted an educational intervention that included a foundational dermoscopy training workshop and subsequent monthly telementoring video conferences. We performed a retrospective observational study to evaluate the impact of this intervention on the number of nevi needed to biopsy to detect a melanoma.
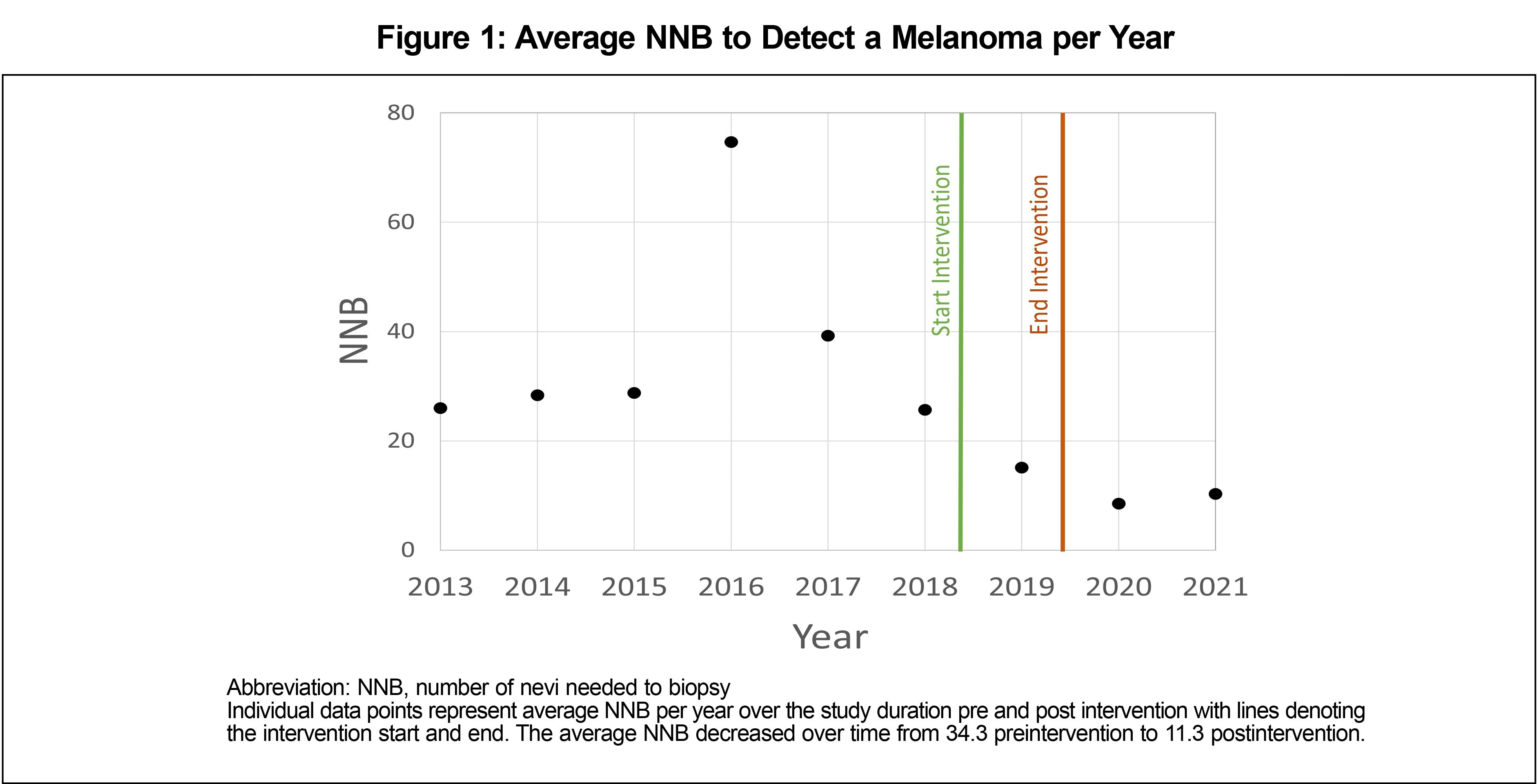
Results: The number of nevi biopsied to detect one melanoma decreased from 34.3 to 11.3 following the training intervention.
Conclusion: Dermoscopy training for primary care practitioners resulted in a significant reduction in the NNB to detect melanoma.
The incidence of melanoma is rising across the United States, with Maine having the tenth highest incidence in the nation.1 This, in combination with a national shortage of dermatologists, means that primary care practitioners (PCPs) must play a pivotal role in early skin cancer detection.2 Studies have shown that PCPs desire more training in dermatology and the evaluation of cutaneous lesions.3-5 To address this need, we offered a dermatology training intervention targeted for PCPs that included a 90-minute Triage Amalgamated Dermoscopic Algorithm (TADA) workshop, subsequent monthly telementoring sessions via the Extension of Community Health Outcomes project (ECHO)6, and equipping participating clinics with dermatoscopes.7
Dermoscopy is a noninvasive tool that allows for visualization of colors and structures in the epidermis and dermis at 10x magnification. In trained hands, dermoscopy improves the user’s ability to differentiate benign melanocytic nevi from melanoma.8 Despite the benefits of dermoscopy, fewer than 15% of PCPs are trained to use a dermoscope.3 The Triage Amalgamated Dermoscopic Algorithm (TADA) was developed to aid novice dermoscopists in the evaluation of skin growths.9,10 TADA training equips learners with the skills needed to differentiate benign from malignant dermoscopic images with high sensitivity and specificity.10 While it is known that the use of dermoscopy reduces the number needed to biopsy (NNB) to detect melanoma, most of this literature is in the dermatology setting.11 Our research aims to test if meaningful reductions in NNB are possible in the primary care setting as well.
This project was reviewed by the Maine Medical Center Institutional Review Board and was deemed exempt. To study the impact of this intervention on melanoma detection in the primary care setting, we developed a skin biopsy database. We then compared the NNB to detect melanoma before and after the educational intervention.
Educational Intervention
Participants were eligible if they were a primary care attending, resident, or advanced practice provider (APP) in family medicine, internal medicine, internal medicine/pediatrics, or pediatrics. Participation was voluntary and no compensation was provided. TADA workshops were taught in-person or virtually 11 times over the course of 8 months to 267 PCPs. The workshop included training on benign and malignant skin growths.8 Following workshop participation, learners were invited to join monthly ECHO telementoring sessions on dermoscopy. Seventeen primary care clinics were provided with dermatoscopes.
Data Extraction and Analysis
Using the current procedural terminology codes, we extracted all skin biopsies performed in our health system by PCPs between November 5, 2013 and November 6, 2021 from our electronic medical record. We conducted a retrospective chart review of the biopsy data. We manually reviewed pathology reports, and we entered results into Research Electronic Data Capture software (REDCap). In this analysis, only biopsy results that revealed nevi or melanoma were included. A total of 1,236 biopsies were in the preintervention group and 1,042 biopsies were in the postintervention group. NNB was used to assess differences in the ratio of biopsies that had nevi versus melanoma outcomes over time. The NNB ratio is calculated by dividing the total number of biopsies (nevi and melanoma) by the number of biopsied melanomas.12 A lower NNB indicates a greater accuracy in differentiating benign and malignant lesions.13 We used a χ2 test of independence to evaluate statistical significance by between biopsy outcome type (melanoma or nevus) and time period (pre- or postintervention).
Preintervention, pathology revealed a total of 1,200 biopsies were nevi and 36 were melanomas. Postintervention, pathology revealed a total of 950 biopsies were nevi and 92 were melanomas. The NNB of nevi to detect a melanoma decreased from 34.3 preintervention to 11.3 postintervention. The χ2 test of independence examining the relationship between biopsy outcome type and time period was significant (χ2 [1, N=11,523]=36.21, P<.001). The average annual NNB results were lower in the postintervention time period than the preintervention time period (Figure 1).
Following dedicated training of PCPs in our health system on the clinical and dermoscopic findings in benign and malignant skin growths, the number of nevi biopsied to detect a melanoma was reduced. While educational interventions do not consistently result in practice change, dermoscopy training, ongoing telementorship, and easy access to a dermatoscope led to widespread dermoscopy use9 and a meaningful reduction in NNB to detect melanoma.
Following the intervention, there was an increase in the rate of biopsies performed and the total number of melanomas detected in the primary care setting. This could be due to multiple factors: improved detection of melanoma due to the use of dermoscopy, increased likelihood to perform biopsies of suspect skin cancers at the primary care clinic (rather than refer to dermatology), and/or expansion of the primary care workforce.
Reduced NNB has implications not only on patient experience, but also on health care costs. Due to climate change, rates of skin cancer in the United States are predicted to increase by 10% by 2050.14 With the rising skin cancer incidence, PCPs need to be equipped with the necessary skills to evaluate skin growths accurately. Reducing the NNB has the potential to improve quality, reduce cost, and foster provider engagement, all important aspects of the quadruple aim.15
However, we must be cautious when interpreting these results, as overdiagnosis of skin cancer is a legitimate concern.16 In the postintervention time period, there was an increase in the number of melanomas being detected in the primary care setting. The goal of early melanoma detection is to reduce melanoma morbidity and mortality. This does come with the potential for detection of slow-growing indolent types of melanomas. However, at present, clinicians do not have reliable bedside diagnostic or FDA-approved genetic tests to aid in determining the metastatic potential of a melanoma. Furthermore, early-stage melanoma is amenable to low-risk outpatient surgery at a reasonable cost.
This study has limitations. The sample size for melanomas was small and the study was conducted in a single health system. Without a control group, we were unable to determine how much of the improvement in biopsy ratios and NNB was due to additional clinical experience. We also have not accounted for changes in skin cancer incidence in our patient population.
Acknowledgments
Financial Support: This study received financial support from the MaineHealth Accountable Care Organization.
References
- U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on 2020 submission data (1999-2018): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; www.cdc.gov/cancer/dataviz, released in June 2021.
- Glazer AM, Farberg AS, Winkelmann RR, Rigel DS. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153(4):322-325. doi:10.1001/jamadermatol.2016.5411
- Williams NM, Marghoob AA, Seiverling E, Usatine R, Tsang D, Jaimes N. Perspectives on dermoscopy in the primary care setting. J Am Board Fam Med. 2020;33(6):1022-1024. doi:10.3122/jabfm.2020.06.200238
- Seiverling E, Ahrns H, Stevens K, et al. Dermoscopic Lotus of Learning: Implementation and Dissemination of a Multimodal Dermoscopy Curriculum for Primary Care. J Med Educ Curric Dev. 2021;8:2382120521989983. Published 2021 Feb 1. doi:10.1177/2382120521989983
- Najmi M, Brown AE, Harrington SR, Farris D, Sepulveda S, Nelson KC. A systematic review and synthesis of qualitative and quantitative studies evaluating provider, patient, and health care system-related barriers to diagnostic skin cancer examinations. Arch Dermatol Res. 2022;314(4):329-340. doi:10.1007/s00403-021-02224-z
- Project ECHO. MaineHealth. 2022. Accessed January 17, 2023. https://www.mainehealth.org/Healthcare-Professionals/Project-ECHO
- Seiverling EV, Li D, Stevens K, Cyr P, Dorr G, Ahrns H. Distance learning and spaced review to complement dermoscopy training for primary care. Dermatol Pract Concept. 2021;11(2):e2021030. Published 2021 Apr 12. doi:10.5826/dpc.1102a30
- Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159(3):669-676. doi:10.1111/j.1365-2133.2008.08713.x
- Seiverling EV, Ahrns HT, Greene A, et al. Teaching benign skin lesions as a strategy to improve the Triage Amalgamated Dermoscopic Algorithm (TADA). J Am Board Fam Med. 2019;32(1):96-102. doi:10.3122/jabfm.2019.01.180049
- Rogers T, Marino ML, Dusza SW, et al. A clinical aid for detecting skin cancer: The Triage Amalgamated Dermoscopic Algorithm (TADA). J Am Board Fam Med. 2016;29(6):694-701. doi:10.3122/jabfm.2016.06.160079
- Nelson KC, Swetter SM, Saboda K, Chen SC, Curiel-Lewandrowski C. Evaluation of the number-needed-to-biopsy metric for the diagnosis of cutaneous melanoma: a systematic review and meta-analysis. JAMA Dermatol. 2019;155(10):1167-1174. doi:10.1001/jamadermatol.2019.1514
- Marchetti MA, Yu A, Nanda J, et al. Number needed to biopsy ratio and diagnostic accuracy for melanoma detection. J Am Acad Dermatol. 2020;83(3):780-787. doi:10.1016/j.jaad.2020.04.109
- Shahwan KT, Kimball AB. Should we leave the skin biopsies to the dermatologists? JAMA Dermatol. 2016;152(4):371-372. doi:10.1001/jamadermatol.2015.5051
- Kaffenberger BH, Shetlar D, Norton SA, Rosenbach M. The effect of climate change on skin disease in North America. J Am Acad Dermatol. 2017;76(1):140-147. doi:10.1016/j.jaad.2016.08.014
- Bodenheimer T, Sinsky C. From triple to quadruple aim: care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-576. PMID:25384822 doi:10.1370/afm.1713
- Welch HG, Mazer BL, Adamson AS. The rapid rise in cutaneous melanoma diagnoses. N Engl J Med. 2021;384(1):72-79. doi:10.1056/NEJMsb2019760


There are no comments for this article.