Introduction: Operating in-person instruction, residential living, and other activities at institutions of higher education (IHEs) in the context of the pandemic of severe acute respiratory syndrome—coronavirus 2 (SARS-CoV-2) have posed a multitude of challenges. Identification of asymptomatic cases at IHEs is crucial, as a large reservoir of virus can potentially develop among students. Unfortunately, despite the advantages, rapid antigen tests (RATs) have variously been shown to perform poorly when used with asymptomatic individuals.
Methods: In order to address the appropriateness of RAT use in screening asymptomatic populations like those at IHEs, we conducted a rapid review of published evaluations of RATs available in the United States, where sensitivity and specificity were reported specifically from asymptomatic populations. We extracted sensitivity and specificity for asymptomatic populations reported in each article, along with location and important notes. The data are presented narratively.
Results: A total of 11 articles were included for evaluation and presentation, representing tests from four manufacturers. Sensitivity ranged from 35.8% to a high of about 71%, with caveats to the higher number about exposure. Both the low and high sensitivity rates were observed in Abbott BinaxNOW RATs. Due to heterogeneity and publishing differences, a meta-analysis was not feasible, but RAT tests in asymptomatic populations tended to identify roughly half of those identified as infected via reverse transcription-polymerase chain reaction. Specificity ranged from 97.8% to 100%.
Conclusion: The results of this rapid review indicate serious issues in misidentifying asymptomatic individuals as COVID-19 negative, when in fact they are infected and carrying the SARS-CoV-2 virus.
Operating in-person instruction, residential living, and other activities at institutions of higher education (IHEs) in the context of the pandemic of severe acute respiratory syndrome–coronavirus 2 (SARS-Cov2) has posed a multitude of challenges. The SARS-CoV-2 virus causes Coronavirus Disease 2019 (COVID-19), a serious and potentially deadly illness. IHEs present a particularly difficult problem, because they mix younger, and potentially asymptomatic, carriers of the virus, with high-density residential circumstances, and frequent interactions with older individuals (instructors, administrators, facility staff, etc) who may be at elevated risk for severe outcomes. The rapid identification of SARS-CoV-2 at the start of instructional periods (eg, academic years or semesters), as well as throughout the period in areas of medium-to-high community transmission, has been crucial to controlling outbreaks.
A key component of infection control at IHEs has been the use of systematic screening and surveillance, achieved by testing regimens. At the time of this writing, the US Centers for Disease Control and Prevention (CDC) recommends testing upon entry, and at least once per week thereafter, of students in moderate-to-high transmission areas, with considerations given to vaccination status and other issues.1 However, implementation of asymptomatic testing can present a massive, previously unbudgeted expense for IHEs, and additionally draws resources from clinical ascertainment of symptomatic patients. Additionally, the de facto gold standard for SARS-CoV-2 infection determination involves reverse-transcriptase polymerase chain reaction (RT-PCR) testing of samples obtained via nasopharyngeal (NPG) swab, which is expensive, resource-intensive, and not often feasible to do rapidly.2 A variety of other methods have arisen in the face of the challenges of RT-PCR testing, including the use of easily-collected salivary PCR-based tests,3 the pooling of samples,4 and the use of rapid antigen tests (RATs) that are inexpensive, potentially self-administered, and generally produce results in 10-15 minutes.5 This combination of low cost, ease of administration, and rapid results have led many institutions to consider using RATs for large-scale, asymptomatic screening.
Identification of asymptomatic cases at IHEs is crucial, as a large reservoir of virus can potentially develop among students. Unfortunately, despite the advantages, RATs have variously been shown to perform poorly when used with asymptomatic individuals. In order to address the appropriateness of RAT use in screening asymptomatic populations like those at IHEs, we conducted a rapid review of published evaluations of RATs, where sensitivity and specificity were reported specifically from asymptomatic populations.
Data Sources/Search Strategy
Our review aimed to collect and assess evaluations of SARS-CoV-2 RATs (a) that are approved for use in the United States, and (b) for which sensitivity and specificity in asymptomatic people have been explicitly reported. We conducted an initial literature search using the PubMed interface of MEDLINE in May 2021-August 2021, using iterations of the term “Covid-19 Rapid Antigen Tests,” and then initiated a rapid, functional review of abstracts and titles for appropriateness. We then repeated our search using Ovid MEDLINE (R) and In-Process, In-Data-Review and Other Non-Indexed Citations 1946 to October 26, 2021 using the search string “[((assessment OR evaluation) AND (COVID OR COVID-19) and (asymptomatic OR presymptomatic) AND ("rapid antigen test" OR "rapid antigen tests" OR rapid antigen OR RAT))].” The literature search was updated again in January 2022, using the same terms in PubMed and OVID.
Inclusion/Exclusion Criteria
At the time of this writing, there are 45 rapid antigen tests that have been granted emergency use authorization (EUA) by the US Food and Drug Administration (FDA). Studies were selected if they (a) evaluated rapid antigen tests that have been granted EUA by the FDA, and (b) were conducted among asymptomatic populations. Any studies conducted outside of the United States were excluded. We also eliminated reports that were duplicative, such as documents first disseminated on preprint servers, that were later published in peer-reviewed journals.
Data Extraction
We extracted sensitivity and specificity for asymptomatic populations reported in each article, along with location and important notes. The data are presented narratively.
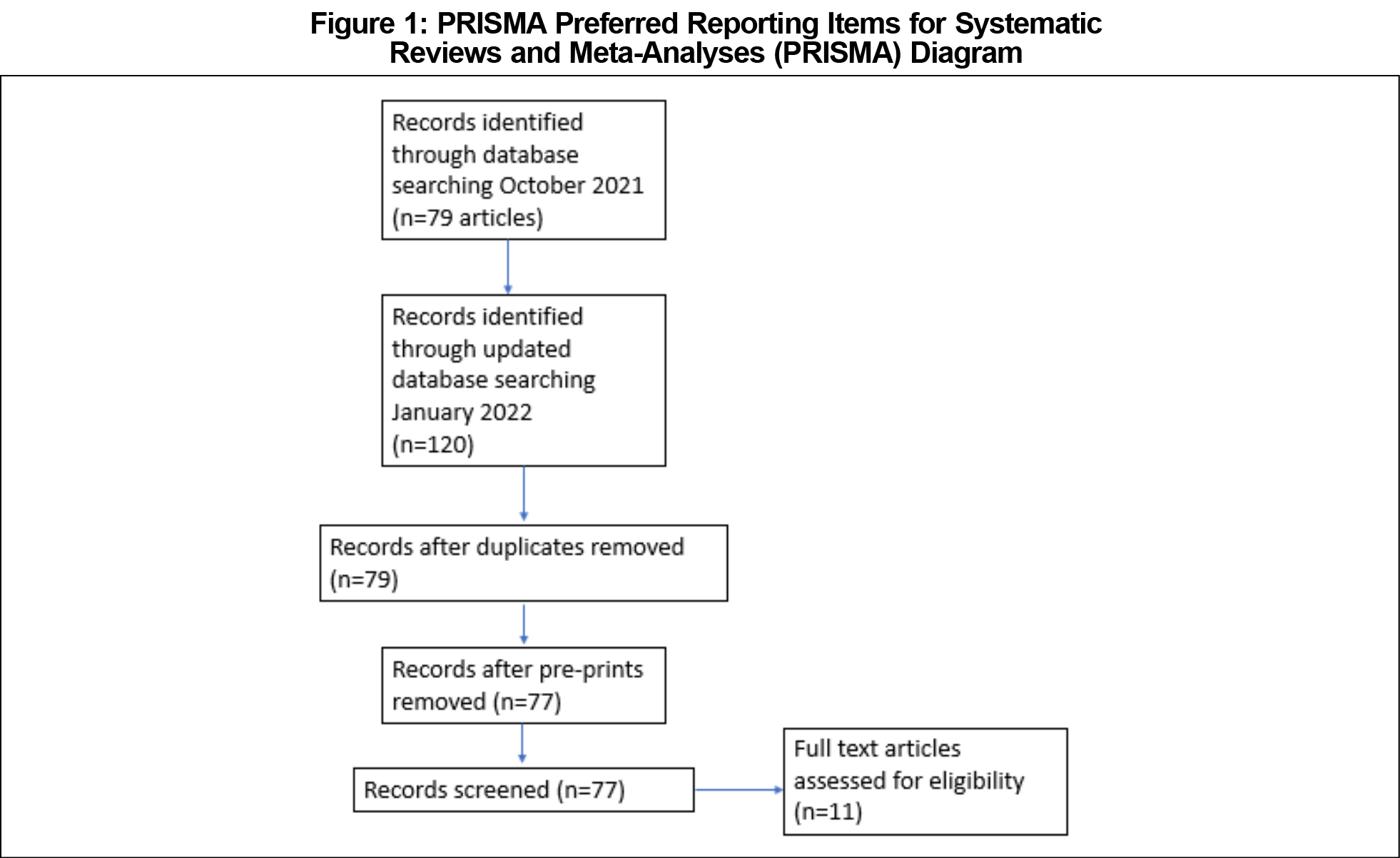
The initial search of identified 61 articles in PubMed with search terms of “COVID-19 rapid antigen tests,” and another 18 articles in Ovid MEDLINE. The update in January 2022 using PubMed produced 54 articles with search terms of “COVID-19 ‘rapid antigen test’ asymptomatic,” and 35 articles with search terms of “COVID-19 ‘rapid antigen tests’ asymptomatic.” Identical terms from the previous MEDLINE via OVID search were used again, which resulted in 31 articles. Out of these 199 articles, 77 articles were eligible for screening, after eliminating duplicate and preprint articles; 11 of these articles, representing tests from four manufacturers, met criteria has having assessed the performance of rapid antigen tests among asymptomatic individuals with COVID-19 within the United States only. 2,5–14 The overall processing of search results is detailed in a PRISMA diagram (Figure 1).
Sensitivity ranged from 35.8% to a high of about 71%, with caveats to the higher number about exposure. Both the low and high sensitivity rates were observed in Abbott BinaxNOW RATs. Due to heterogeneity and publishing differences, a meta-analysis was not feasible, but RAT tests in asymptomatic populations tended to identify roughly half of those identified as infected via RT-PCR. Specificity ranged from 97.8% to 100%. The results are fully displayed in Table 1.
A challenge of rapid antigen tests usage among asymptomatic individuals includes the low sensitivity of the tests and the need for confirmation tests with RT-PCR tests as recommended by the FDA’s EUA. The results of this rapid review indicate serious issues in misidentifying asymptomatic individuals as COVID-19 negative, when in fact they are infected and carrying the SARS-CoV-2 virus. The implication in a college population is twofold: infections among individuals living in congregate settings are potentially missed; and a false negative test may lead to an assumption that one is infection-free, and lead such individuals to behave as though they are infection-free in a college setting, where group living, social gatherings, in-class attendance, and events are frequent parts of daily life.
Although RATs appear to have sensitivity issues in asymptomatic individuals, there is still a benefit to using them for detecting and monitoring COVID-19 infections. A major advantage of RATs is the speed of their results compared to RT-PCR tests. This is especially useful for screening in the case RT-PCR tests are unavailable.12 As such, RATs may be useful in identifying those who are actually infectious, and may help screen individuals at the start of an acute event or gathering. Additionally, RATs have proven successful in detecting the emergent Omicron variant.15 There is also utility in frequent use of RATs to identify those who have a high enough viral load for detection via this modality.16 However, few if any institutions are using RATs with the daily or constant frequency required for this approach. Finally, new approaches to antigen testing may yield better results in asymptomatic populations in the future.17
This rapid review should be viewed with several limitations in mind. First, in order to share results quickly, we relied upon one review pass, and did not search grey literature or other databases beyond the two main interfaces that access MEDLINE. However, the results we observed are consistent, and we believe it is unlikely that a report exists outside of MEDLINE that would contradict the fundamental concerns about RAT sensitivity revealed in the 11 articles included in this rapid review. It is also notable that sensitivity results have reported in the peer-reviewed literature for only four RATs, to our knowledge, representing a small minority of the 45 RATs available in the United States within the timeframe of this review. Additionally, we do not believe that a meta-analysis would be feasible, given variations in reporting style and populations across the 11 articles. In this case, we believe that the narratively-presented results speak for themselves. Additionally, there are reports that fell outside of our inclusion/exclusion criteria that nonetheless replicate the findings we report here.18,19
In summary, we have deep concerns about the use of RATs for broad, weekly screening in asymptomatic populations, such as within IHEs, as they have a high probability of missing infected individuals due to low sensitivity. Pooled sampling techniques that allow for quicker and more efficient use of RT-PCR based testing are a much better alternative if one is needed.20
References
- Guidance for Institutions of Higher Education (IHEs). Centers for Disease Control and Prevention. Published February 7, 2022. Accessed February 9, 2022. https://www.cdc.gov/coronavirus/2019-ncov/community/colleges-universities/considerations.html
- Pilarowski G, Lebel P, Sunshine S, et al. Performance characteristics of a rapid severe acute respiratory syndrome coronavirus 2 antigen detection assay at a public plaza testing site in San Francisco. J Infect Dis. 2021;223(7):1139-1144. doi:10.1093/infdis/jiaa802
- Takeuchi Y, Furuchi M, Kamimoto A, Honda K, Matsumura H, Kobayashi R. Saliva-based PCR tests for SARS-CoV-2 detection. J Oral Sci. 2020;62(3):350-351. doi:10.2334/josnusd.20-0267
- Interim Guidance for Use of Pooling Procedures in SARS-CoV-2 Diagnostic and Screening Testing. Centers for Disease Control and Prevention. Published February 11, 2020. Accessed February 9, 2022. https://www.cdc.gov/coronavirus/2019-ncov/lab/pooling-procedures.html
- Stokes NL, Reed KA, Berbari EF, Vetter S, Binnicker MJ. Evaluation of the BinaxNOW COVID-19 rapid antigen test in an asymptomatic patient population undergoing preprocedural screening. J Clin Microbiol. 2021;59(12):e0165021. doi:10.1128/JCM.01650-21
- Pollock NR, Jacobs JR, Tran K, et al. Performance and implementation evaluation of the Abbott BinaxNOW rapid antigen test in a high-throughput drive-through community testing site in Massachusetts. J Clin Microbiol. 2021;59(5):e00083-21. doi:10.1128/JCM.00083-21
- Prince-Guerra JL, Almendares O, Nolen LD, et al. Evaluation of Abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection at two community-based testing sites - Pima County, Arizona, November 3-17, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(3):100-105. doi:10.15585/mmwr.mm7003e3
- Pollock NR, Tran K, Jacobs JR, et al. Performance and operational evaluation of the Access Bio CareStart rapid antigen test in a high-throughput drive-through community testings in Massachusetts. Open Forum Infect Dis. 2021;8(7):ofab243. doi:10.1093/ofid/ofab243
- Sood N, Shetgiri R, Rodriguez A, et al. Evaluation of the Abbott BinaxNOW rapid antigen test for SARS-CoV-2 infection in children: implications for screening in a school setting. PLoS One. 2021;16(4):e0249710. doi:10.1371/journal.pone.0249710
- Okoye NC, Barker AP, Curtis K, et al. Performance characteristics of BinaxNOW COVID-19 antigen card for screening asymptomatic individuals in a university setting. J Clin Microbiol. 2021;59(4):e03282-e20. doi:10.1128/JCM.03282-20
- James AE, Gulley T, Kothari A, Holder K, Garner K, Patil N. Performance of the BinaxNOW coronavirus disease 2019 (COVID-19) Antigen Card test relative to the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) real-time reverse transcriptase polymerase chain reaction (rRT-PCR) assay among symptomatic and asymptomatic healthcare employees. Infect Control Hosp Epidemiol. 2022;43(1):99-101. doi:10.1017/ice.2021.20
- Siddiqui ZK, Chaudhary M, Robinson ML, et al; CONQUER COVID Consortium. Implementation and accuracy of BinaxNOW rapid antigen COVID-19 test in asymptomatic and symptomatic populations in a high-volume self-referred testing site. Microbiol Spectr. 2021;9(3):e0100821. doi:10.1128/Spectrum.01008-21
- Harris DT, Badowski M, Jernigan B, et al. SARS-CoV-2 rapid antigen testing of symptomatic and asymptomatic individuals on the University of Arizona Campus. Biomedicines. 2021;9(5):539. doi:10.3390/biomedicines9050539
- Pray IW, Ford L, Cole D, et al; CDC COVID-19 Surge Laboratory Group. Performance of an antigen-based test for asymptomatic and symptomatic SARS-CoV-2 testing at two university campuses - Wisconsin, September-October 2020. MMWR Morb Mortal Wkly Rep. 2021;69(5152):1642-1647. doi:10.15585/mmwr.mm695152a3
- Omicron Variant: What You Need to Know. Centers for Disease Control and Prevention. Published February 2, 2022. Accessed February 9, 2022. https://www.cdc.gov/coronavirus/2019-ncov/variants/omicron-variant.html
- Kahn R, Holmdahl I, Reddy S, Jernigan J, Mina MJ, Slayton RB. Mathematical modeling to inform vaccination strategies and testing approaches for COVID-19 in nursing homes. Clin Infect Dis Off Publ Infect Dis Soc Am. Published online June 4, 2021:ciab517. doi:10.1093/cid/ciab517
- Chen H, Li Z, Feng S, et al. Femtomolar SARS-CoV-2 antigen detection using the microbubbling digital assay with smartphone readout enables antigen burden quantitation and tracking. Clin Chem. 2021;68(1):230-239. doi:10.1093/clinchem/hvab158
- Eshghifar N, Busheri A, Shrestha R, Beqaj S. Evaluation of analytical performance of seven rapid antigen detection kits for detection of SARS-CoV-2 Virus. Int J Gen Med. 2021;14:435-440. doi:10.2147/IJGM.S297762
- McKay SL, Tobolowsky FA, Moritz ED, et al; CDC Infection Prevention and Control Team and the CDC COVID-19 Surge Laboratory Group. Performance evaluation of serial SARS-CoV-2 rapid antigen testing during a nursing home outbreak. Ann Intern Med. 2021;174(7):945-951. doi:10.7326/M21-0422
- Miller EW, Lamberson CM, Akabari RR, et al. Development and validation of two quantitative RT-PCR diagnostic assays for detecting severe acute respiratory syndrome cronavirus 2 genomic targets across two specimen types. J Mol Diagn JMD. Published online February 4, 2022:S1525-1578(22)00018-6. doi:10.1016/j.jmoldx.2021.12.010



There are no comments for this article.